|
THE LATEST PUBLISHED RESEARCH
Ground-Breaking Gene Therapy Performed for Leber's Congenital Amaurosis
Two studies published online by The New England Journal of Medicine represent the first successful use of gene therapy in humans to combat retinal dystrophy caused by mutations in the gene encoding retinal pigment epithelium (RPE)-specific 65-kDa protein (RPE65), a form of Leber's Congenital Amaurosis (LCA).
In one of the studies, three young adults received subretinal injections of recombinant adeno-associated virus vector 2/2 expressing RPE65 complementary DNA under the control of a human RPE65 promoter. In each patient, the eye with the worst acuity was selected as the study eye, and the contralateral eye was used as a control. Patients with visual acuity in the study eye better than 20/120 were excluded. Pre-injection assessments of visual function and immune status were repeated at two, four, six and 12 months after administration of the vector. (Two of the three patients have not yet reached the 12-month point.)
Investigators reported that this gene therapy was not associated with immediate adverse effects and can lead to modest improvements in visual function, even in patients with advanced degeneration. No clinically significant change in visual acuity or peripheral visual fields occurred, and no change in retinal responses on electroretinography were detected. However, one patient experienced significant improvement in visual function on microperimetry and dark-adapted perimetry. The same patient also showed improvement in a subjective test of visual mobility.
The investigators pointed out that it will take several years to determine whether further retinal degeneration is delayed in these patients. They also wrote that their findings support further clinical studies of this experimental approach in other patients with mutant RPE65, particularly children, who may be more likely to benefit than adults.
The second study also evaluated the safety and preliminary efficacy of subretinal delivery of a recombinant adeno-associated virus carrying RPE65 complementary DNA in three patients. The patients have been followed for five months. Investigators used a surfactant to reduce adsorption of viral particles to the contact surfaces of delivery devices. They also processed the vector to remove empty capsid with the goal of increasing the potency and minimizing immunogenicity to capsid proteins. They did not use a tissue-specific promoter. For each patient, the eye with worse function was selected for delivery of the adeno-associated virus.
After treatment, pupillary light reflex testing confirmed an increase in retinal sensitivity for all three eyes. Treated eyes became approximately three times as sensitive to light as they were at baseline, and the sensitivity surpassed that of the (previously better functioning) other eye. Improvements in pupillary light reflexes were accompanied by increases in visual acuity. Average logMAR visual acuity improved by 0.28 for one patient (p<0.001), by 0.45 for one patient (p<0.001), and by 0.34 for one patient (p=0.002), more than 3.5 lines.
The investigators also reported a trend toward improvement in the visual-field area of all patients. Improvements exceeded the variation measured in the fellow eyes. Improvements in nystagmus were also noted for all three patients, and one patient showed improved ability to navigate an obstacle course.
No local or systemic adverse related to the vector occurred. One patient developed a macular hole two weeks after treatment. The patient was unaware of it, and it did not expand at subsequent visits. The authors of the paper wrote that although the follow-up was short and the patients did not achieve normal vision, the study provides the basis for further gene therapy studies in patients with LCA.
Sources: Bainbridge JWB, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008;358. DOI: 10.1056/NEJMoa0802268. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008;358. DOI: 10.1056/NEJMoa0802315.
Autofluorescence Reveals New Information about Two Retinal Conditions
Two recently published studies indicated that autofluorescence photography reveals characteristics of multifocal choroiditis and panuveitis (MCP) and birdshot chorioretinopathy (BSCR) that other imaging modalities do not. One of the studies was a retrospective review of 36 eyes of 18 consecutive patients with MCP evaluated in a retinal referral practice. Patients had a comprehensive exam, including color fundus photography and angiography. Autofluorescence photography was performed with an excitation filter with bandpass wavelengths of 535 to 585 nm and a barrier filter with a bandpass of 615 to 715 nm.
Autofluorescence showed more widespread involvement of the RPE than would be suspected by other imaging methods. Usually, but not always, chorioretinal hypoautofluorescent spots greater than or equal to 125 µm had the clinically evident correlate of a punched-out scar visible by fundus photography. Chorioretinal hypoautofluorescent spots smaller than 125 µm, which could number in the hundreds, typically were not visible by fundus photography. All chorioretinal scars visible by fundus photography were visible by autofluorescence. During follow-up, many patients developed new clinically evident chorioretinal scars that were presaged in autofluorescence photographs. Of the 36 eyes in the study, 23 had CNV, which was readily visible because of its hyperautofluorescent boundaries.
In another study (a retrospective, observational case series published online), researchers investigated the fundus autofluorescence characteristics of BSCR in 16 eyes of eight consecutive patients in a referral practice. In 11 eyes (69 percent), hypoautofluorescent regions indicated RPE atrophy, which was hard to distinguish by other imaging methods. Some of the hypoautofluorescent regions corresponded to the hypopigmented birdshot lesions, but others did not, suggesting that the choroid and the RPE can be affected independently. Eight eyes (50 percent) showed linear hypoautofluorescent streaks along the retinal vessels, most of which corresponded to visible changes at the level of the RPE. Placoid hypoautofluorescence in the macula was seen in six eyes and was correlated significantly with best-corrected visual acuity of 20/50 or less (p<0.05), leading investigators to conclude that RPE atrophy in the macula may be an important cause of poor central visual acuity in BSCR.
Sources: Haen SP, Spaide RF. Fundus autofluorescence in multifocal choroiditis and panuveitis. Am J Ophthalmol 2008;145(5):847-853. Koizumi H, Pozzoni MC, Spaide RF. Fundus autofluorescence in birdshot chorioretinopathy. Ophthalmology 2008;115(5):e15-e20.
Estrogen Exposure and Risk of AMD
Postmenopausal hormone (PMH) and oral contraceptive (OC) use may be associated with a lower risk of neovascular AMD, according to results of a study by Brigham and Women's Hospital and Harvard Medical School. Researchers assessed PMH use, past use of OCs, ages at menarche and menopause and parity among 74,996 postmenopausal women. Over 22 years, they identified cases of early AMD (554 patients) and neovascular AMD (334 patients) with visual acuity of 20/30 or worse. They used Cox models to calculate the relative risk for each exposure, adjusted for smoking and other factors.
Current PMH users had a 48 percent lower risk of neovascular AMD compared with those who had never used PMH, although risk did not decline linearly with longer durations of use. Risk was lowest for PMH users who had used OCs in the past. In contrast, risk of early AMD was 34 percent higher among current PMH users, and OC use was unassociated with risk. The only remarkable finding for the endogenous estrogenic factors was a 26 percent lower risk of early AMD for women who had given birth one or more times.
Source: Feskanich D, Cho E, Schaumberg DA, et al. Menopausal and reproductive factors and risk of age-related macular degeneration. Arch Ophthalmol 2008;126(4):519-24.
Study Finds Endophthalmitis Rare Following Anti-VEGF Injection in a Community Setting
In a retrospective interventional case series involving 10,254 intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents in a community setting, the incidence of suspected endophthalmitis was 0.029 percent (95 percent confidence interval, 0.006-0.085 percent). The injections were performed as office-based procedures from January 5, 2005 to October 18, 2007 [406 pegaptanib (Macugen), 3,501 bevacizumab (Avastin), 6,347 ranibizumab (Lucentis)]. Povidone-iodine was used as part of the preinjection preparation; preinjection antibiotics, eye drape or surgical attire were not used.
No cases of culture-proven endophthalmitis and three cases of suspected endophthalmitis occurred. All three patients with suspected endophthalmitis regained preinjection visual acuity. No difference in risk was found between bevacizumab and ranibizumab (p=.6).
Source: Pilli S, Kotsolis A, Spaide RF, et al. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol 2008;145(5):879-882.
Two-Year FOCUS Results Published
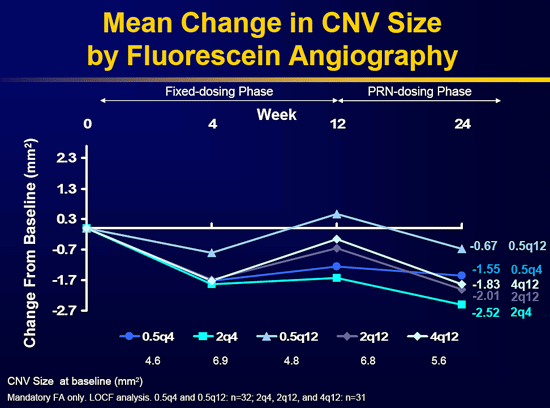
Two-year results from the FOCUS study were published in the May issue of the American Journal of Ophthalmology. FOCUS was a multicenter, randomized, single-masked, controlled study to assess the safety and efficacy of combined treatment with ranibizumab and verteporfin (Visudyne) photodynamic therapy (PDT) in patients with predominantly classic CNV secondary to neovascular AMD. Through two years, ranibizumab plus PDT was more effective than PDT alone and was associated with a low rate of adverse events. Also, the visual acuity benefit of adding ranibizumab to PDT in year one persisted through year two.
At month 24, 88 percent of patients receiving combined therapy lost fewer than 15 letters of visual acuity from baseline compared with 75 percent of patients who received PDT alone. Twenty-five percent of the combination therapy group gained 15 or more letters compared with seven percent of the PDT-alone group. The difference in mean visual acuity change between the two groups was 12.4 letters (p<.05 for all between-group differences). On average, ranibizumab plus PDT patients exhibited less lesion growth and greater reduction of CNV leakage and subretinal fluid accumulation. Also, they required fewer PDT retreatments than patients who received PDT alone (mean 0.4 vs. 3.0).
Endophthalmitis occurred in 2.9 percent of the combination group, and serious intraocular inflammation occurred in 12.4 percent. Endophthalmitis and serious intraocular inflammation occurred in 0 percent of the PDT-alone group. Incidences of serious nonocular adverse events were similar in the two treatment groups.
Patients in the study received monthly intravitreal injections of ranibizumab 0.5 mg (n=106) or sham injections (n=56). All patients received PDT on day zero and then quarterly as needed.
Source: Antoszyk AN, Tuomi L, Chung CY, Singh A, FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol 2008;145(5):862-874.
|