US Pharm.
2007;32(4):HS29-HS39.
Complex
regional pain syndrome (CRPS) is one of the most perplexing and debilitating
disorders facing health care professionals involved with pain management. The
exact incidence is unknown, and the lack of precise diagnostic criteria has
hampered efforts to improve research involving therapeutic interventions.
The disorder was originally
reported in the medical literature after the Civil War and was described as a
syndrome of pain, autonomic disturbances, involuntary movements, and changes
in the skin or hair in an extremity with a nerve injury. This constellation of
symptoms was originally named causalgia. Early descriptions of the
pathophysiology of the disorder in the 1940s emphasized the involvement of an
overactive sympathetic nervous system–leading to the diagnosis of reflex
sympathetic dystrophy. Other names used for this disorder include
algodystrophy, shoulder–hand syndrome, posttraumatic
dystrophy, and Sudeck's atrophy.1
Diagnosis
The identification
of the syndrome remains problematic. Current consensus guidelines have
established diagnostic criteria (Table 1). Pain and hyperesthesia, an
increased sensitivity or awareness of pain, are present in almost all patients
with CRPS. Pain is usually described by these patients as neuropathic in
nature and having the characteristics of burning, pricking, or shooting. Most
patients describe the location of the pain as deep within the affected limb.
2,3 Up to one half of patients will experience deficits in temperature
and tactile perception on examination.2

Autonomic symptoms may include
swelling or edema, skin color changes, abnormal sweating, or alterations in
skin temperature. More than half of patients experience edema in the affected
limb, and the skin temperature difference between limbs is often greater than
1°C. Early in the course of the syndrome, patients are likely to report edema
and a warm extremity. As the disease progresses, edema usually regresses and
the patient will often report a cooler extremity. Skin may appear to be paler,
or even discolored to a blue or purple undertone.2
Motor symptoms vary widely
among patients. In one study, more than 75% of the patients reported weakness
in the affected limb, and 50% reported tremor.3 Patients are also
found to have decreased range of motion and may eventually experience
contractures after disuse of the affected limb.2 In severe cases,
patients may even lose perception of limb positioning to the point of ignoring
the impacted limb. This particular symptom is known as limb neglect.4
Patients often notice decreased hair and nail growth as well as thinning of
the skin with time.2
Incidence and Risk Factors
Very little is known about the
incidence of the disorder. In fact, up to 10% of patients will not be able to
identify an event or injury that precipitated their symptoms.5
Although the disorder has been reported in children, the usual age of onset is
between 36 and 46 years. The majority of cases occur in women. The most common
inciting events include surgery, fracture, casting or immobilization, sprain,
crush injury, and stroke with motor involvement.2
The majority of patients with
CRPS are not able to continue with regular employment, and many of them
require significant assistance with normal household responsibilities such as
cooking, cleaning, and laundry.6
Pathophysiology
In most cases, an
inciting event such as trauma or immobilization leads to the release of
proinflammatory neurotransmitters such as substance P and prostaglandins. This
normal response to pain transmits the stimuli to the spinal cord to initiate a
pain response. As with many injuries, these mediators start the initial warmth
and swelling or edema at the site.3 Up until this point, the pain
response is characterized as a normal physiologic one.
The mystery of this disorder
centers on the perpetuation of this response into a vicious cycle of pain and
disuse of the affected limb. Two theories are proposed in the medical
literature for CRPS. The first is that the mechanism causing the release of
the inflammatory mediators does not terminate appropriately. The second theory
proposes that the clearance of the inflammation mediators is altered and that
they exist in the periphery for prolonged periods. In either case, an excess
of these substances sensitizes the nervous system, both peripherally and
centrally, to the perception of pain. This perpetual painful stimulation is
thought to increase sympathetic tone in the area in response to increased
levels of epinephrine and other constricting catecholamines. Eventually, this
increase in sympathetic drive leads to vasoconstriction, cooling of the
extremity, and possibly atrophy.3 Patients naturally avoid painful
stimuli through decreased use and guarding of the limb. Disuse further
decreases the clearance of catecholamines and impairs venous drainage of edema
in the area.7 In one study, depression and emotional impairment
related to pain associated with increased levels of catecholamines.8
X-rays of limbs impacted by CRPS often show osteoporotic changes in the bone
even as quickly as four to eight weeks after symptoms appear.9
Psychiatric Comorbidity
A long-running
controversy surrounding CRPS and its treatment involves its association with
psychiatric illness. Patients with CRPS commonly experience depression,
anxiety, and phobias with a prevalence that ranges from 18% to 64%. The
incidence of individual psychiatric disorders within this patient population,
however, does not differ significantly from the chronic pain patient
population.10 The severity of depression in CRPS has been linked to
the severity of associated pain, which may indicate a physiologic or
neurochemical link between the two conditions.8 In an evaluation of
a CRPS population, Lynch and colleagues found no evidence that previous
psychiatric illness predisposed to the development of CRPS.11
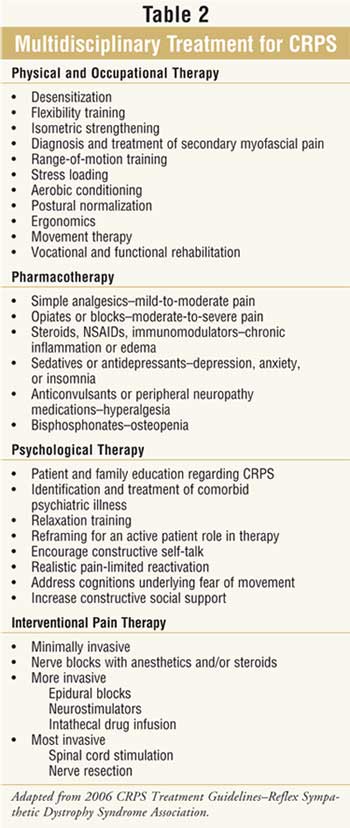
Nonpharmacologic Therapy
Therapy including
physiotherapy, psychological counseling, behavioral therapy, and potentially
neurostimulation are key to success in the management of CRPS (Table 2
). Lack of multidisciplinary care often results in poor outcomes; however,
finding clinicians with knowledge and experience specific to this disorder can
be difficult. Obtaining insurance company reimbursement for these
interventions can also be problematic.8
Physiotherapy is usually directed at
gradual restoration of normal sensory function. This process is accomplished
through occupational and physical therapy. Examples of successful therapy
include progressing from gentle movement of the affected limb to weight
bearing and then on to normal activities. Another example for sensory therapy
may involve initial therapy with textures such as silk and increasing to
rougher textures, and incorporating temperature changes into therapy.12
In one study retrospectively examining 145 cases, those who received physical
therapy reported significantly less pain and a better functional status.13
Successful therapy is most likely to occur with effective pain management
through pharmacologic intervention; see discussion below. Trigger point
injections of anesthetics or pain medications may facilitate therapy with
range of motion exercises. The goal of this rehabilitation should be
normalization of activity or an ergonomically adapted environment to improve
functionality.2
Behavioral therapy is less familiar
to many clinicians, but it is a key part of therapy for these patients. One
example is trained relaxation of muscle groups. This training begins with
unaffected muscle groups and gradually proceeds to the affected limbs. In
controlled evaluations, this practice has demonstrated the benefit of reducing
the spread of symptoms. Another behavioral technique emphasizes the importance
of patient involvement in the treatment process. This procedure, known as
"reframing of symptoms as a call to action," prevents passivity on the part of
the patient. Being actively engaged and involved in treatment is associated
with more positive outcomes.8 Current consensus guidelines for CRPS
recommend that patient's with symptom duration greater than two months should
have a psychological evaluation.2 The combination of psychological
and behavioral therapy may help to break the interaction between physical pain
and stress behaviors, prevent disuse of the extremity, and encourage positive
coping skills as a response to pain.8 The medical literature,
including a meta-analysis, supports this approach for chronic pain patients
but has not been evaluated in CRPS.14
Neurostimulation is a more invasive
technique reserved for more severe cases. Both peripheral and spinal cord
stimulation have been studied for these patients. These interventions are
generally monitored by an interventional pain specialist and considered after
failure of less invasive modalities.2
Pharmacologic Therapy
As with
nonpharmacologic interventions, pharmacologic therapy for CRPS often requires
implementing multiple types of therapy simultaneously. This therapy may
include interventional procedures such as nerve blocks and regional injections
of anesthetics, steroid therapy, therapy for osteoporosis, traditional
neuropathic pain medications, and opiates. At the time of writing, no
pharmacologic therapies were approved by the FDA for treatment of CRPS
specifically. Experimental therapies for treating the disorder, however, are
under evaluation.
Nerve blocks are utilized for CRPS
patients for temporary pain relief and to facilitate compliance with physical
and occupational therapy. These nerve blocks are typically administered in the
medical office setting or as an outpatient surgery procedure by a trained
interventional pain specialist. Although no controlled medical evidence exists
to support their use in specific situations, they are one of the most commonly
employed interventions for these patients. Trials evaluating the use of blocks
often do so in isolation from other therapies. For example, in 2004 a study
conducted by Taskaynatan et al. found no benefit to blocks containing
lidocaine and steroids when compared to placebo. However, this study examined
blocks as an isolated treatment and not in conjunction with other modalities,
such as physical therapy.15 Different types of blocks are
administered depending on the patient's symptoms and the area affected by
CRPS. Sympathetic blocks, intravenous regional blocks, and somatic nerve
blocks are all utilized for symptom management.2 These injections
often include an anesthetic as well as steroids and, in some cases, agents to
block sympathetic outflow.
Systemic therapy with steroids early
in the course of CRPS has shown benefit in randomized trials. In one study
evaluating 30 mg of prednisolone for 12 weeks in comparison to placebo,
patients treated with the steroids experienced less pain over the study
duration.16 A study comparing prednisolone 40 mg to an active
control group, piroxicam 20 mg, in CRPS related to stroke demonstrated
positive outcomes for both pain and quality of life (QOL) for the
steroid-treated patients. The group receiving piroxicam did not show any
significant change in either pain or QOL over the study period.17
Based on the available information, steroids should be considered early in
management of CRPS to improve long-term outcomes.
As discussed earlier, CRPS is
associated with osteoporosis in the affected limb. The bisphosphonate
pamidronate has been evaluated in placebo-controlled trials in this
population. Although long-term studies have not been conducted, short-term
evaluations indicate improvements in pain scores and overall disease severity.
Small but statistically significant improvements have been documented for this
intervention to improve physical functionality in patients.18
Therapy with calcitonin has also been evaluated in short-term, nonblinded
trials. Calcitonin combined with physical therapy was no better than physical
therapy alone in a two-month evaluation of pain scales, trophic changes, and
range of motion.19
The most common pharmacologic
therapies utilized for these patients are medications for neuropathic pain. As
with other interventions for CRPS, very little randomized or controlled data
exist to support their use. Many large neuropathic pain trials excluded
patients with CRPS. Most evidence to support their use is anecdotal.20
In a placebo-controlled trial evaluating gabapentin, pain scores improved
initially but returned to baseline. Sensory deficits, measured by monofilament
exams, improved in the gabapentin-treated groups. Patients in these groups
were significantly more likely to report dizziness and somnolence associated
with therapy.21
Trials involving patients with CRPS
are difficult to conduct. Enrollment is complicated by controversy regarding
the diagnostic criteria as well as the heterogeneous nature of the symptoms.
Evaluation of these trials is difficult because most do not value an
integrated or multidisciplinary care team. For a therapy to be considered
effective, it is generally accepted that pain scores should improve at least
50%,22 a degree of improvement rarely documented in interventions
evaluating one type of therapy.
Two interventions have shown
promise. The first was demonstrated in stroke patients, in whom occupational
and physical therapy initiated in the first two to three days after stroke
reduced the risk of developing CRPS. Administration of 500 mg of vitamin C for
50 days after wrist fracture and immobilization reduced the incidence of CRPS
from 22% to 7% in a placebo-controlled evaluation.23
Complex regional pain syndrome
is a debilitating disorder involving a sustained pain response after nerve
injury. Successful therapy for the disorder should include pharmacologic as
well as nonpharmacologic interventions aimed at returning the patient to a
functional status.
REFERENCES
1. Grabow TS, Christo PJ, Raja SN. Complex regional pain syndrome: diagnostic controversies, psychological dysfunction, and emerging concepts. Adv Psychosom Med. 2004;25:89-101.
2. Ghai B, Durega GP. Complex regional pain syndrome: a review. J Postgrad Med. October-December 2004;50:300-307.
3. Birklein F. Complex regional pain syndrome. J Neurol. 2005;252:131-138.
4. Frettloh J, Huppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. 2006;124:184-189.
5. Veldman PH, Reynen HM, Arntz IE, et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012-1016.
6. Kemler MA, Furnee CA. The impact of chronic pain on life in the household. J Pain Symptom Manage. 2002;23:433-441.
7. Pearce JM. Chronic regional pain and chronic pain syndromes. Spinal Cor. 2005;43:263-268.
8. Bruehl S, Chung OY. Psychological and behavioral aspects of complex regional pain syndrome management. Clin J Pain.2006;22:430-437.
9. Birklein F, Hanwerker HO. Complex regional pain syndrome: how to resolve the complexity? Pain. 2001;94:1-6.
10. Bruehl S, Carlson CR. Predisposing psychological factors in the development of reflex sympathetic dystrophy. Clin J Pain. 1992;49:337-347.
11. Lynch ME. Psychological aspects of reflex symptathetic dystrophy: a review of the adult and paediatric literature. Pain. 1992;49:337-347.
12. Harden RN, Swan M, King A, et al. Treatment of complex regional pain syndrome: functional restoration. Clin J Pain. 2006;22:420-424.
13. Birklein F, Reidle B, Sieweke N, et al. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101:262-269.
14. Morley S, Eccleston C, Williams A. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy and behavior therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1-13.
15. Taskaynatan MA, Ozgul A, Tan AK, et al. Bier block with methylprednisolone and lidocaine in CRPS type I: a randomized, double-blinded, placebo-controlled study. Reg Anesth Pain Med . 2004;29:408-412.
16. Christensen K, Jensen EM, Noer I. The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scan. 1982;148:653-655.
17. Kalita J, Vajpayee A, Misra UK. Comparison of prednisolone with piroxicam in complex regional pain syndrome following stroke: a randomized controlled trial. QJM. 2006;99:89-95.
18. Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med. 2004;5:276-280.
19. Sahin F, Yilmaz F, Kotevoglu N, et al. Efficacy of salmon calcitonin in complex regional pain syndrome (Type I) in addition to physical therapy. Clin Rheumatol. 2006;25:143-148.
20. Rowbotham MC. Pharmacologic management of complex regional pain syndrome. Clin J Pain. 2006;22:425-429.
21. van de Vusse AC, Stomp-van den Berg SG, Kessels A, et al. Ranomised controlled trial of gabapentin in Complex Regional Pain Syndrome type I. BMC Neurol. 2004;4:13.
22. Forouzanfar T, Weber WE, Kemler M, et al. What is a meaningful pain reduction in patients with complex regional pain syndrome type I. Clin J Pain. 2003;19:281-285.
23. Quisel A, Gill JM, Witherell P.
Complex regional pain syndrome: which treatments show promise? J Fam Prac
. 2005;54:599-603.
To comment on this article, contact
[email protected].